The mission of the National Abortion Federation is to
unite, represent, serve, and support abortion providers in delivering patient-centered, evidence-based care.
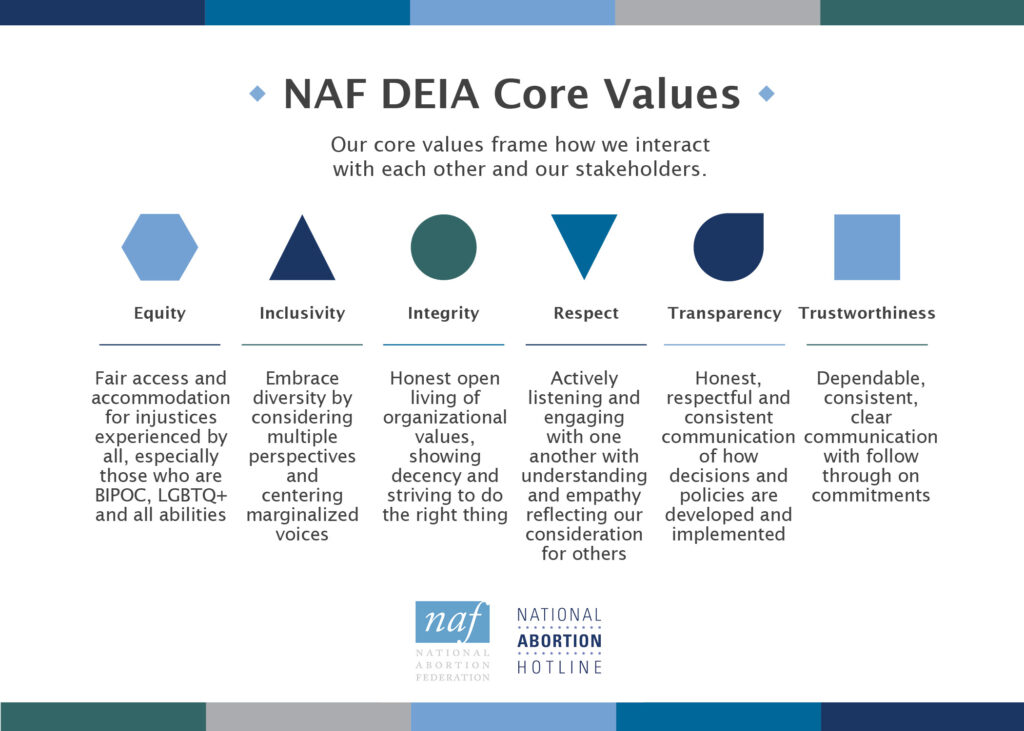
Core Values
Organizational Core Values are the principles and fundamental beliefs that guide our daily functioning. Through collaboratively defining and implementing these values, we establish a foundation that allows us to maintain a workplace dedicated to continual learning and prepared to recognize and address oppressions and micro-aggressions.
Fair access and accommodation for injustices experienced by all, especially those who are BIPOC, LGBTQ+, and all abilities
Embrace diversity by considering multiple perspectives and centering marginalized voices
Honest open living of organizational values, showing decency and striving to do the right thing
Actively listening and engaging with one another with understanding and empathy reflecting our consideration for others
Honest, respectful and consistent communication of how decisions and policies are developed and implemented
Dependable, consistent, clear communication with follow through on commitments
NAF's Leadership

Brittany Fonteno
President & CEO

Melissa Fowler
Chief Program Officer

Julie Gonen
Chief Legal Officer

Veronica E. Jones
Chief Operating Officer
NAF's Board of Directors

Melissa Grant
carafem
Board Chair

Leah Coplon CNM, MPH
Abortion on Demand

Kersha Deibel, MPH, MSW
Planned Parenthood Federation of America

Justin T. Diedrich, MD, MSCI, FACOG
Eden Surgical Center

Tiffany Hailstorks, MD, MPH
Emory University School of Medicine

Curtiss P.S. Hannum, MSN, APN, CRNP
The Women's Centers

Erin King, MD
Hope Clinic

Tammi Kromenaker
Red River Women's Clinic

John C. Markley, MD, PhD
UCSF School of Medicine

Sarah McNeil, MD
TEACH Program

Tram Nguyen, MHA/MBA
Planned Parenthood Federation of America

Catherine Obando MSN, NP-C

TaRhonda Slydell, BSN, MBA-HCA
Presidential Women’s Center

Maria Mercedes Vivas, MD, MPH
Fundacion Orientame

Lin-Fan Wang, MD
Family Practice Physician

Ying Zhang, MD, MPH
University of Washington Department of Family Medicine

Rachel K. Jones, PhD
Guttmacher Institute Liaison

Beverly Winikoff, MD, MPH
Gynuity Health Projects Liaison
